ICMR-Central Ethics Committee on Human Research (CECHR)
| Central Ethics Committee on Human Research (CECHR) Common forms | View Forms |
| Notification | |
Background
- Multidisciplinary and multi-sectoral in composition, and functions as per ICMR National Ethical Guidelines for Biomedical and Health Research Involving Human Participants, 2017.
- Appointed by Director General, ICMR to work at the national level for development and updating of National Ethical Guidelines, to address emerging ethical aspects of Biomedical and Health Research, to guide ICMR Ethics Policy, review research of national importance with complex ethical issues being led by ICMR or referred to it by the government ministries and departments.
- ICMR Bioethics Unit, Bengaluru serves as the Secretariat for the CECHR.
- CECHR is registered with the Office for Human Research Protections (OHRP) under the U.S. Department of Health and Human Services (No. IRB00012875, Dated 7th Dec 2020) to facilitate ICMR-NIH collaborative research.
- CECHR is registered with the National Ethics Committee Registry for Biomedical and Health Research (NECRBHR), Department of Health Research (Reg. no EC/NEW/INST/2021/1879 Dated 9 July 2021)
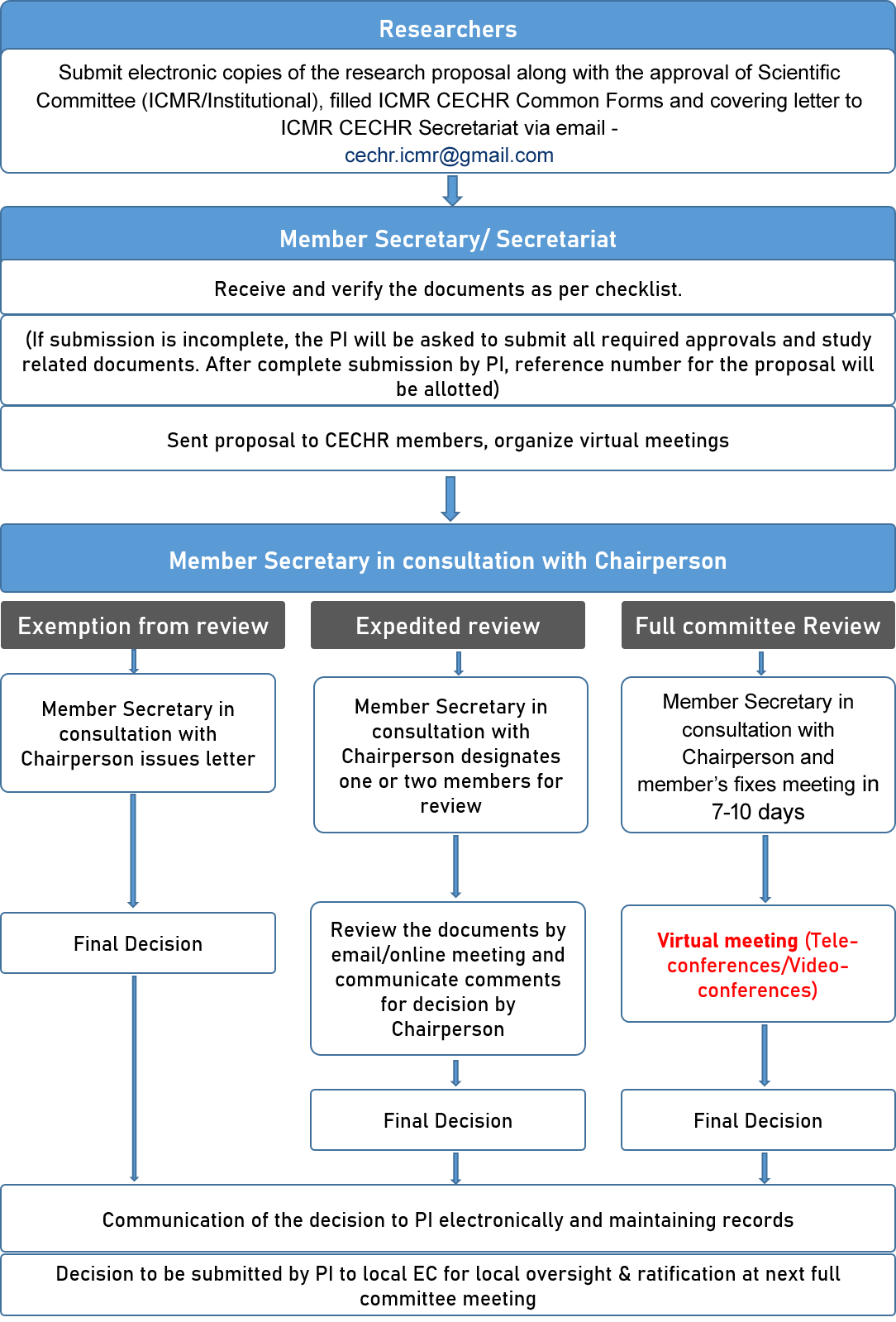
PROCEDURES FOR EC REVIEW & RESPONSIBILITIES