ICMR COVID 19 National Ethics Committee
The global pandemic of Novel Coronavirus COVID 19 necessitates appropriate research to inform public health actions, and ICMR its network of institutions plans to conduct a number of such studies. Rapid and robust ethics review is needed to safeguard the rights, safety, welfare and dignity of the research participants. In this regard, Director General, ICMR has constituted a special ethics committee ICMR COVID 19 National Ethics Committee (CoNEC) to undertake high priority quality expedited ethics review of COVID 19 research.
Scope and Purpose
CoNEC would conduct ethics review of COVID 19 related biomedical and health research coordinated or led by ICMR or its institutions across the country. Review of clinical trials (for marketing approvals) should be in accordance to New Drugs and Clinical Trial Rules, 2019.
CoNEC Members

Procedures for EC Review and Responsibilities
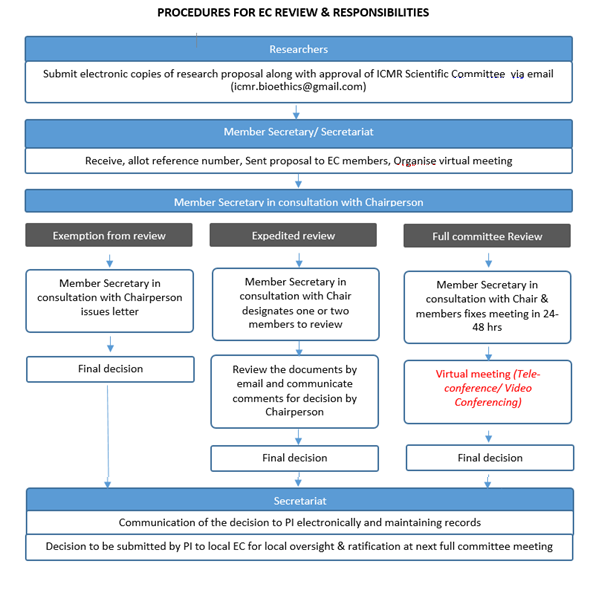
For more information, contact icmr.bioethics@gmail.com

